Evidence of Gate-Opening on Xenon Adsorption on ZIF-8 An Adsorption and Computer Simulation Study
Abstract
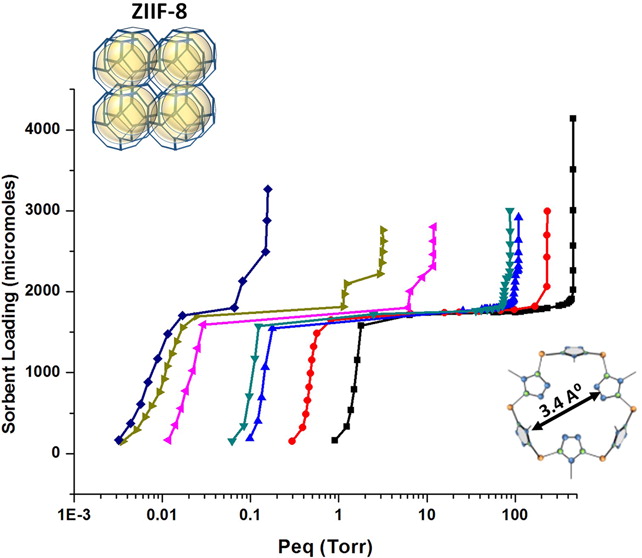
We report on experimental (thermodynamics and kinetics) and computer simulation results for Xe sorption in ZIF-8. At temperatures below ∼145 K, there are two substeps present in adsorption isotherms (before saturation is reached). The substep that occurs at higher loadings was identified as corresponding to ZIF-8’s gate-opening transition. Above 145 K, this higher-loading substep is no longer resolvable. We determined the isosteric heat of adsorption for this system and obtained a value of 244 meV for sorption on the more strongly binding sites in the ZIF-8. We found that there is a peak in the isosteric heat of adsorption, as a function of sorbent loading, associated with the gate-opening transition. We estimated the heat of transition for gate-opening to have an upper bound of ∼30 meV. Our results for the isotherms and the isosteric heats are compared with those from our Monte Carlo computer simulations, obtained using both the structure of ZIF-8 before and after the gate-opening transition and a new set of mixed Lennard-Jones parameters. We conducted measurements for the sorption kinetics for this system. We found that, while the sorption occurs faster as the loading increases before the gate-opening transition, the equilibration times increase with loading in the gate-opening region, resulting in an unusual nonmonotonic behavior for the variation of this quantity as a function of sorbent loading.