Carbon monoxide adsorption in ZIF-8- Kinetics and equilibrium
Abstract
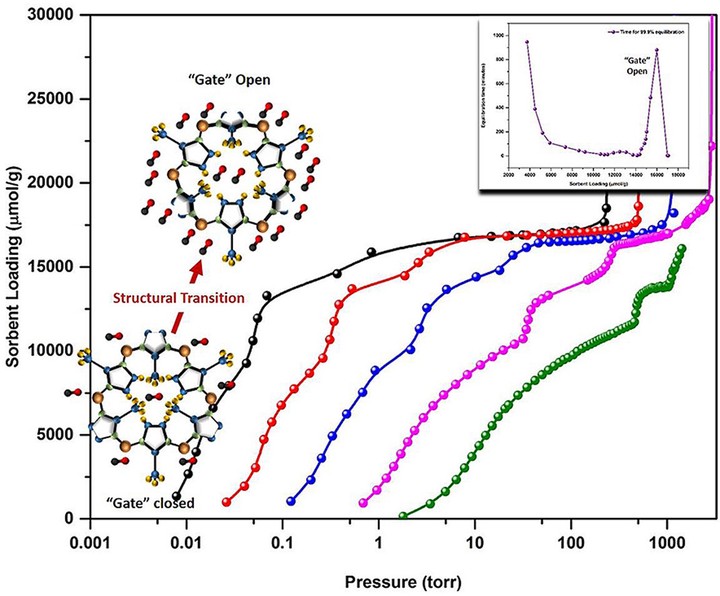
We present the results of a study of the kinetics and equilibrium characteristics of CO sorption in ZIF-8. We measured adsorption isotherms at five temperatures between 72.67 and 104.58 K; all the isotherms exhibit three sub-steps below saturation. We have studied the evolution of the adsorption kinetics of this system by monitoring the equilibration times as a function of sorbent loading. We find that there is a very large, sharp maximum, in the equilibration times in the region corresponding to the highest loading sub-step in the sorption data; and we find a much smaller local maximum for loadings corresponding to the intermediate-loading sub-step. We have determined the isosteric heat of adsorption for CO in ZIF-8 and its dependence on sorbent-loading. We find a sharp isosteric heat of adsorption peak at values of the sorbent-loading corresponding to the highest loading isotherm sub-step. We discuss a possible explanation for the combined results of kinetic and isosteric results for this system. We have also studied the temperature evolution of the characteristics of the sub-steps present in the isotherms. We find that, as was the case for other systems in ZIF-8, the relative pressures at which the two higher-loading isotherm sub-steps occur move increasingly closer to the saturated vapor pressure as the isotherm temperatures increase.